Title: The deconstruction of a polymeric solvation cage: a critical promotion strategy for PEO-based all-solid polymer electrolytes
Authors: Ruiyang Li, Haiming Hua, Xueying Yang, Jianling Tian, Qichen Chen, Rongwei Huang, Xue Li, Peng Zhang* and Jinbao Zhao*
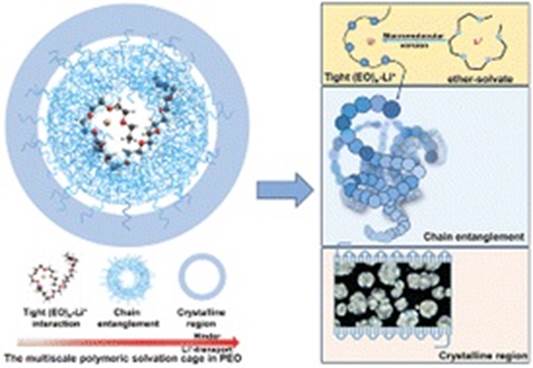
Abstract: Solid polymer electrolytes (SPEs) are considered one of the potential alternative solutions to address the safety issues of lithium-ion batteries (LIBs) induced by liquid electrolytes. Poly(ethylene oxide) (PEO) as one of the most representative polymer matrix can solvate and transport lithium ions and has been studied since the 1970s. However, its unsatisfactory solid-state ion-transport behavior at room temperature still greatly limits its commercialization. Not entirely the same as the ionic conduction in the liquid electrolyte, ionic conduction in SPEs is trapped by the multi-scale structural effects of chain entanglement at the macroscopic scale and a tight (EO)n–Li+ chelating effect at the microscopic scale. These two effects are coupled together, acting like a cage for Li+ conduction, thus inducing a low ionic conductivity (σ) and Li+ transference number (tLi+). Herein, the deconstruction of this polymeric solvation cage via a multi-scale ionic conduction promotion strategy is proposed for the first time via the introduction of 1,1,2,2-tetrafluoroethyl-2,2,3,3-tetrafluoropropylether (TTE) and poly(ethylene oxide carbonates) (SPE1100). TTE could loosen the polymer chain entanglement and decouple the influence of structural effects at different scales but without solvating Li+. As a result, SPE1100 with a carbonyl group could compete for Li+ coordination with (EO)n, modify the tight (EO)n–Li+ chelating interaction, and then successfully improve the Li+-transport behavior of PEO-based SPEs, with both increased σ and tLi+ (3.2 × 10−4 S cm−1 and 0.57 at 25 °C). Herein, we firstly probed the ionic conduction mechanism of PEO-based SPEs on the basis of the coupled structural effects at both the macromolecular and micromolecular scale. The strategy of decoupling the mutual influence between scales and then solving the bottleneck at a separate scale might provide a beneficial approach to fundamentally improve the ion-transport performance of SPEs.
Full-Link: https://pubs.rsc.org/en/content/articlelanding/2024/ee/d4ee01188k