报告题目:Single-Molecule and Single-Active-Site Studies of Stereocontrol
by Chemisorbed Chiral Molecules
报告人: Prof. Peter McBreen
Laval University, Canada
时间: 12月1日(周四)上午9:00
地点: 卢嘉锡楼202报告厅
以下是学术报告的摘要部分:
Abstract:
This talk will describe experimental surface studies of individual bimolecular complexes formed between chiral and prochiral molecules on Pt(111). The work is inspired by the catalysis literature on the enantioselective hydrogenation of ketones and ketoesters on chirally-modified Pt catalysts. Using scanning tunneling microscopy (STM) supported by density functional theory (DFT), we probe non-covalently bonded chemisorption complexes formed between representative prochiral substrates and (R)-(+)-1-(1-naphthyl)ethylamine, (R)-NEA. In all of the systems studied, multiple complexation configurations are populated around the chiral center. Comparison to DFT-predicted structures is used to determine the hierarchy of chemisorption and, sometimes multiple, H-bonding interactions operating in complexes. Sub-molecularly resolved STM images permit the determination of the prochiral ratio (pr) proper to specific locations around the stereogenic center, and the overall pr can, in some cases, be compared to literature values for the enantiomeric ratio (er) for hydrogenation using the same modifier/substrate pair. Time-lapsed STM measurements are used to explore the fluxional properties of individual complexes. The STM experiments provide access to the preorganization step required for the emergence of enantioselectivity and the DFT calculations suggest future approaches to gaining kinetic information on state-specific hydrogenation.
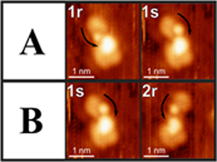
Figure 1. Consecutive STM images of an individual TFAP/(R)-NEA complex taken at a frame rate of one per 52.6 s. The prochirality of TFAP, pro-R or pro-S, is indicated by r or s, respectively.
欢迎有兴趣的老师和同学参加!
固体表面物理化学国家重点实验室
biwn必赢
2016年11月29日
