报告题目:Making polymers from elemental sulfur - and applications in mercury capture
报告人:Dr. Tom Hasell, Royal Society University Research Fellow, Department of Chemistry, University of Liverpool
时间:2019-03-18 10:00
地点:卢嘉锡楼202报告厅
报告摘要:
Sulfur is an industrial by-product, removed as an impurity in oil-refining. This has led to vast unwanted stockpiles of sulfur, and resulted in low bulk prices. Sulfur is therefore a promising alternative feedstock to carbon for polymeric materials. Sulfur normally exists as S8 rings – a small molecule with poor physical properties. On heating, these sulfur rings can open and polymerise to form long chains. However, because of the reversibility of sulfur bonds, these polymers are not stable, and decompose back to S8 over time, even at room temperature. Inverse vulcanisation has made possible the production of high sulfur polymers, stabilised against depolymerisation by crosslinking.[1,2] These high sulfur-materials show excellent potential as low cost water filters to remove mercury.[2-5] Heavy metal contamination exists in the waste streams of many industries, and mercury is of particular concern for human health. Alternative crosslinkers for inverse vulcanisation, from industrial by-products or bio-renewable sources, can be used to reduce the cost and improve the properties of the resultant polymers.[4] Polymers made from sulfur also have many other intriguing properties and applications in optics, electronics, insulation, and antimicrobial materials. We have recently discovered a catalytic route that reduces the required reaction time, temperature, and by-products – as well as allowing otherwise unreactive crosslinkers to be used.[6]
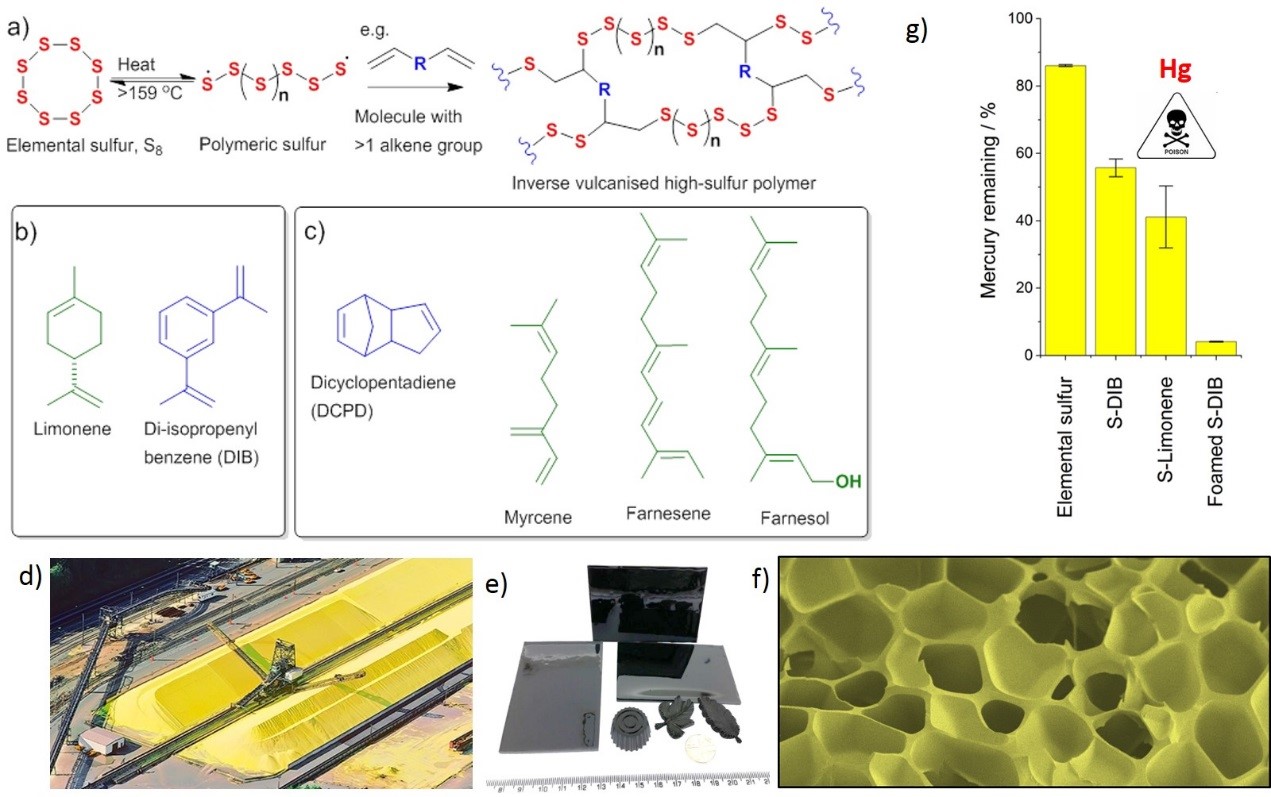
Figure 1: a) Reaction of elemental sulfur and organic crosslinkers produces stable polymers with up to 80 weight % sulfur. b) Previously reported crosslinkers, di-isopropenyl benzene[1] and limonene[2]. c) Alternative crosslinkers reported here.[4] This allows excess elemental sulfur waste, d), to be converted to functional materials, e). These high sulfur polymers can then be foamed in supercritical CO2 to increase the accessible surface, as shown by SEM, f), which significantly increases their ability to capture mercury from solution, g).[3]
[1] W. J. Chung, et al., A., “The use of elemental sulfur as an alternative feedstock for polymeric materials” Nature Chem. 2013, 5, 518–524.
[2] A. M. Crockett, et al., “Sulfur-Limonene Polysulfide: A Material Synthesized Entirely from Industrial By-Products and Its Use in Removing Toxic Metals from Water and Soil” Angew. Chem., Int. Ed., 2016, 55, 1714-1718.
[3] T. Hasell, D. J. Parker, H. Jones, T McAllister, and S. M. Howdle, “Porous inverse vulcanised polymers for mercury capture” Chem.Commun., 2016, 52, 5383 - 5386.
[4] D. J. Parker, H. A. Jones, S. Petcher, L. Cervini, J. M. Griffin, R. Akhtar and T. Hasell “Low cost and renewable sulfur-polymers by inverse vulcanisation, and their potential for mercury capture” J. Mat. Chem. A. 2017,5, 11682-11692
[5] J.-S. M. Lee, D. J. Parker, A. I. Cooper and T. Hasell*, “High surface area sulfur-doped microporous carbons from inverse vulcanised polymers” Journal of Materials Chemistry A, 2017, 5, 18603
[6] X. Wu, J. A. Smith, S. Petcher, B. Zhang, D. J. Parker, J. M. Griffin, and T. Hasell,“Catalytic inverse vulcanisation”, Nature Communications, 2019, 10, 647
报告人简介:
Tom is a Royal Society University Research Fellow in the School of Chemistry at the University of Liverpool. Originally from Yorkshire, he is a graduate of the University of Nottingham, and has spent time researching in America and Japan. Before getting his fellowship, he worked as a postdoc and then research coordinator in Andrew Cooper’s group at Liverpool, where played a significant role in the development of Porous Organic Cages. Tom has worked in a wide range of areas across materials science, including synthesis, supercritical processing, polymer science, nanocomposites, and porous materials. Tom was awarded the European Young Chemist of the year award in 2014. His group is currently working on making functional materials from elemental sulfur. You can find out more about Tom and his research here: https://www.liverpool.ac.uk/chemistry/staff/thomas-hasell/

